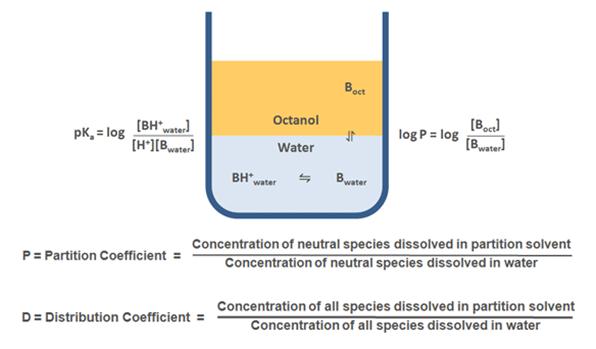
A certain balance of lipophilicity and hydrophilicity is required for a successful drug candidate. Water solubility is essential for a drug to be dissolved in plasma and other aqueous biological fluids, whereas lipophilicity is indispensable for penetrating through biological membranes and is of high importance for the compounds aimed for oral dosing. A widely used model to access the physicochemical properties of the compound is LogP (partition coefficient). Lipophilicity is typically accessed as the distribution of the tested compound between two solvents – typically non-aqueous organic (n-octanol) and aqueous (pH-buffered water), and then LogP is expressed as a log10 of the concentration ratio between two phases. LogP is widely used in cheminformatics and is a component of Lipinski’s “rule of five”, which is a golden standard to evaluate a drug-likeness of a compound. According to this rule, the successful drug candidate should possess a LogP value not greater than 5.
LogD is a distribution coefficient widely used to measure the lipophilicity of ionizable compounds, where the partition is a function of the pH. For non-ionizable compounds LogP = LogD throughout pH range, whereas for ionizable compounds LogD takes into account the partition of both ionized and non-ionized forms. LogD is more convenient for practical measurements, as it takes into account solution pH, which is important for the analysis of the drug candidate properties in various biologic media with different pH values.
Note: Pricing for this service refers to logD determination at one selected pH. For non-charged compounds (at the pH used for logD measurements) logD equals logP, and there is no extra charge for logP measurement. For compounds with known pKa values, price for logP determination will be equal to the price of logD determination; the same applies for the cases where in silico calculated pKa values are used. Pricing for the experimental pKa measurements is subject to discussion and depends on the properties of the tested compounds.
Deliverable
Calculations of the partition ratios (peak area of the analyte in octanol phase to the peak area of the analyte in PBS buffer). A full study report is provided.
Sample Requirement/Submission
A minimal, accurately weighable quantity of dry compound (~1 mg or 2 µmol) or 50 µL of 10-20 mM stock DMSO solution is required for this assay. For multiple assays, a lesser amount of compound per assay may be sufficient, depending on the particular project. We do not need to know the structures of the molecules for ADME testing. However, brutto formulas have to be provided for all studies involving MS detection.
LogD/LogP is one of our ADME tests for physicochemical properties evaluation:
- Aqueous Solubility
- Shake flask solubility using Millipore or pION Solubility Filter Plates (UV or MS-MS detection)
- Laser nephelometry
- Chemical Stability Assay
- Determining pKa
For more information, please contact us info@bienta.net