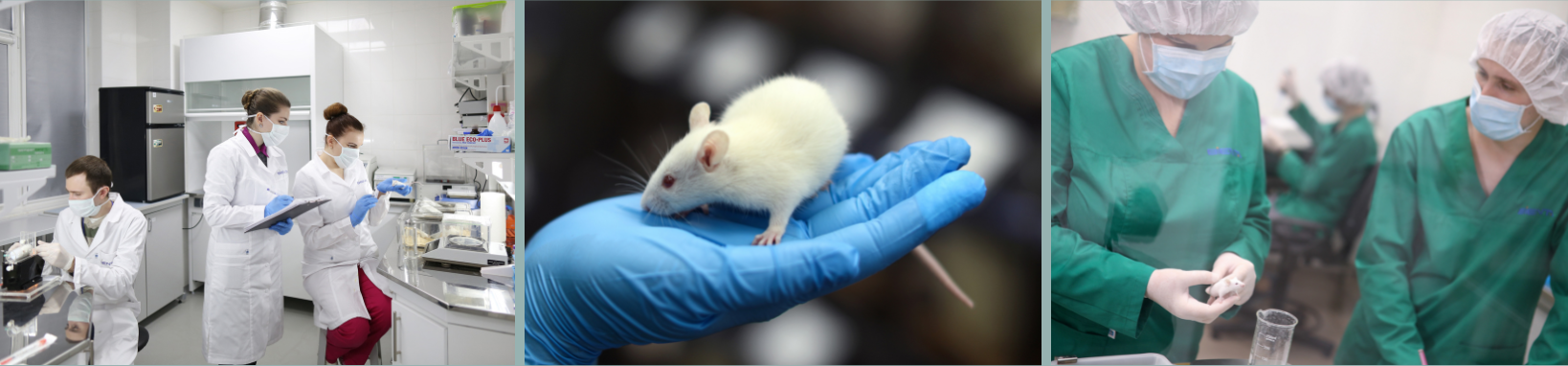
Pharmacokinetic (PK) studies are part of our ADMET panel. The study design can significantly vary depending on the goals and parameters of the tested compounds. We offer PK in widely used mouse inbred (C57BL/6, BALB/c), outbred (CD1, NMRI) strains, and Wistar, or Sprague Dawley rats. All study protocols are reviewed by Bienta’s Institutional Animal Care and Use Committee (BACUC).
A typical PK study in mice involves two drug delivery routes (e.g. PO and IV), 6 time points for each route (for example 5, 15, 30, 60, 120, 240 min for IV and 15, 30, 60, 120, 240 and 360 min for PO) and 4 animals per each time point group/route, plus the common control plasma of Vehicle dosed group, 50 animals in total. We will prepare blood plasma samples and measure compound concentrations by LC-MS/MS using AB Sciex API4000/3000 mass spectrometers and Shimadzu (Prominence, VP) HPLC systems.
We offer:
-
Measurements of various pharmacokinetic and pharmacodynamics parameters;
-
Compound absorption/elimination dynamics following different routes of administration;
-
Biodistribution (brain, liver, kidney, lung, spleen, heart, liquor, etc.);
-
Metabolism/biotransformation – metabolite ID services;
-
Compound excretion dynamics (urine/feces, bile);
-
End-point or serial sampling;
-
Single compound or cassette pharmacokinetics for a mix of 2-3-4-5 compounds;
-
Wide range of time points;
-
Native or perfused tissue sampling;
-
Development of optimized drug delivery formulations to improve the bioavailability (Formulation Screen)
Deliverable: A detailed study report including a complete description of the study design, analytical method, measured test article concentrations, all common PK parameters, and PK graphs. Raw experimental data are available upon request.
Sample Submission. Dry compound or compound in a pre-made animal dosing formulation. Amounts depend on the dosing levels. For a PK study in mice at 10 mg/kg PO and 10 mg/kg IV, about 16 mg of the compound is required. We do not need to know the structures of the molecules for ADME testing. However, brutto formulas have to be provided for all studies involving MS detection.
For more information, please contact us info@bienta.net.